BCI implant’s trial success prefigures ‘new era’ in eye care


An electronic eye implant is giving hope to individuals who have experienced visual impairment due to dry age-related macular degeneration (dry AMD), with 84% of patients in a pivotal trial able to read again once fitted with the device.
Developed by Science Corporation, 38 patients involved in the PRIMAvera trial (NCT04676854) with dry AMD were fitted with the clinical stage company’s PRIMA brain computer interface (BCI) system.

Discover B2B Marketing That Performs
Combine business intelligence and editorial excellence to reach engaged professionals across 36 leading media platforms.
Find out more
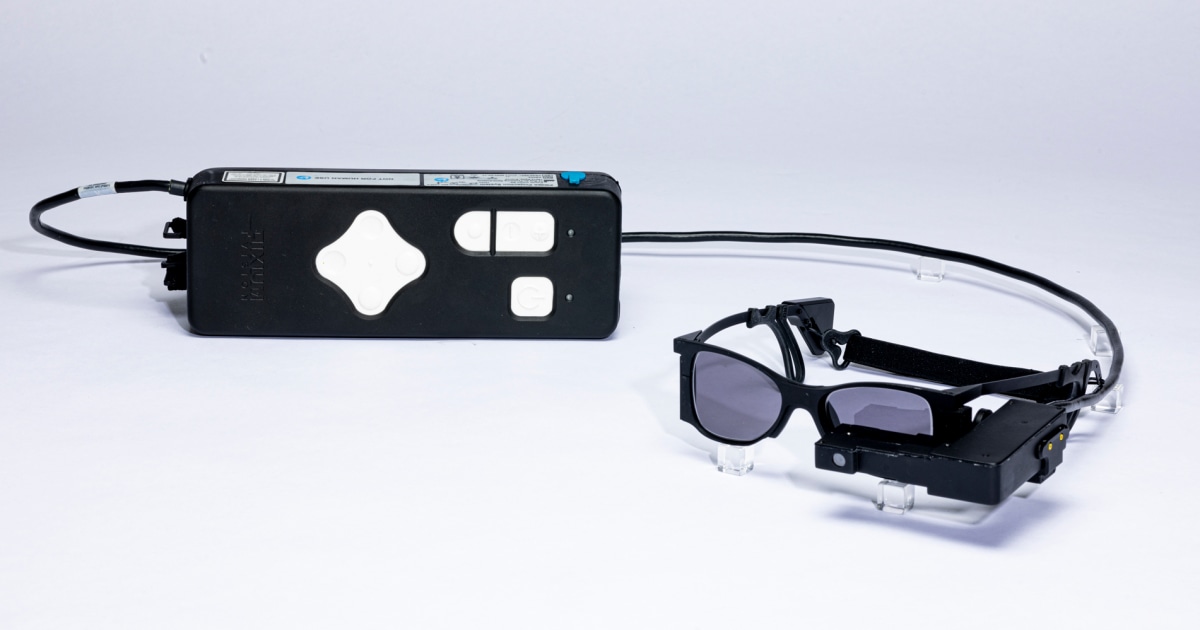
PRIMA is comprised of an ultra-thin microchip implanted under the retina and a visual processing computer worn on a patient’s waistband. Using augmented reality (AR) glasses that contain a video camera and are connected to the computer, artificial intelligence (AI) within the computer converts information into electrical signals. Passed through the retinal and optical nerve cells into the brain, the information is then interpreted as vision.
Each patient in the trial has undergone a rehabilitation programme, teaching them how to interpret the signals and learn how to read using the modality.
Sharing her experience of being fitted with the system, Sheila Irvine, a patient enrolled at trial site Moorfields Eye Hospital, London, said: “I was an avid bookworm, and I wanted that back. I was nervous, excited, all those things. There was no pain during the operation, but you’re still aware of what’s happening.
“It’s a new way of looking through your eyes, and it was dead exciting when I began seeing a letter. It’s not simple, learning to read again, but the more hours I put in, the more I manage to pick up.”
Dry AMD affects around 170 million people globally. The condition, which is currently incurable, causes a gradual loss of central vision by thinning the macula, the part of the eye that sees fine detail, with those affected eventually unable to engage in activities such as reading and driving. While there is a wealth of approved pharmaceutical products for the disease, such treatments only stop progression and are not be able to restore vision.
Mahi Muqit, senior vitreoretinal consultant at Moorfields Eye Hospital and the Institute of Ophthalmology at UCL commented: “In the history of artificial vision, this represents a new era. Blind patients are actually able to have meaningful central vision restoration, which has never been done before.
“Getting back the ability to read is a major improvement in their quality of life, lifts their mood and helps to restore their confidence and independence. The PRIMA chip operation can safely be performed by any trained vitreoretinal surgeon in under two hours – that is key for allowing all blind patients to have access to this new medical therapy.”
In November 2024, the Data Safety Monitoring Board for the study recommended PRIMA for European market approval, concluding that its benefits to patients outweighed the risk of the implantation surgery. Regulatory approval for the system is currently underway in Europe, with Science Corporation anticipating a market launch in 2026. A regulatory filing for PRIMA with the US Food and Drug Administration (FDA) is currently being processed.
link


/countries/china/38488.tmb-1200v.jpg?sfvrsn=b842f_4)